You have been invited to participate in a research study. Before you decide to take part, it is important for you to understand what it will involve and why the research is being done. Please read the following information carefully and ask us for clarification on anything that is unclear. Take all the time you need to decide if you wish to take part.
As we age, our cognitive functions (or our abilities to think, learn and remember) naturally become slower - this is a normal part of the ageing process. However for some people, this process can be more pronounced. Mild Cognitive Impairment (MCI) describes a condition where a person is performing worse cognitively than the “average” person in their age group, but this does not interfere with most regular daily activities.
MCI is associated with a higher risk for developing dementia, a condition characterised by a very significant loss of cognitive functions and independence. However, not all people with MCI necessarily progress to dementia.
Recent research has revealed that a lot can be done to slow down cognitive decline by adopting healthier lifestyle habits: being physically active, sleeping well, eating well and challenging our brain (i.e., “training” it) on a regular basis.
Lifestyle interventions for reducing cognitive decline are starting to become available at some in-person clinics, but delivering them to everyone in need is not easy. Not all geographical regions have the same access to clinics, and not all clinics have the capacity to offer these types of services.
Five Lives, a health technology company, has developed a digital lifestyle and brain-training app called Five Lives MED that may help older people improve their cognition from the comfort of their own home, anywhere in the world. The purpose of this research is to investigate the effectiveness of the Five Lives MED app in improving cognitive function in people with MCI.
You have been identified as a potential participant by your local NHS trust or research register (such as Joint Dementia Research or Dementia Platform UK) in which you are registered.
To take part in the study, you must:
You will not be able to take part if you:
No, you decide if you would like to take part and can have as much time as you wish to consider the information presented to you. You can also withdraw from the study at any time, without giving a reason and without it affecting your medical care. If you choose to withdraw, your data until the point of withdrawal will still be used for data analysis purposes unless you specifically ask us not to use these data. If you do provide a reason for withdrawing, it will be recorded by the investigator.
You will be offered reimbursement for your travel expenses, up to £30 per participant per study visit.
If you choose to participate in this research, informed consent will be taken through a written consent form that the investigator will provide to you. Once this consent has been obtained, the study can begin.
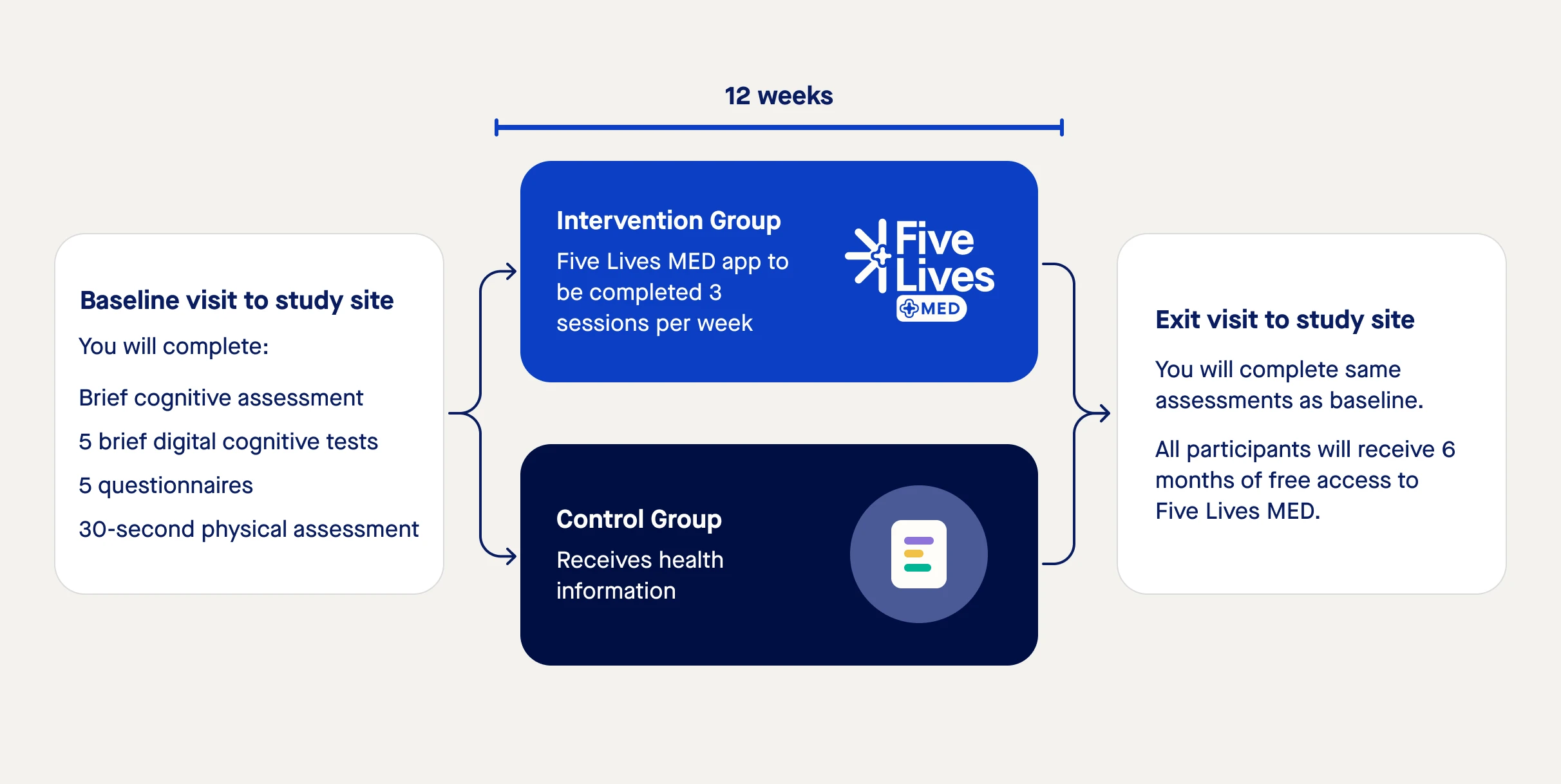
The study will take place over 12 weeks and includes a visit to your study site once at the beginning and once at the end. During each of the two study site visits (lasting about 2 hours), you will complete a set of assessments and questionnaires concerning:
At the end of the first visit, you will be randomly assigned into an intervention group or control group. Those in the intervention group will use the Five Lives MED app for the duration of the 12 weeks and those in the control group will be given standard health and lifestyle information.
See Study Diagram below.
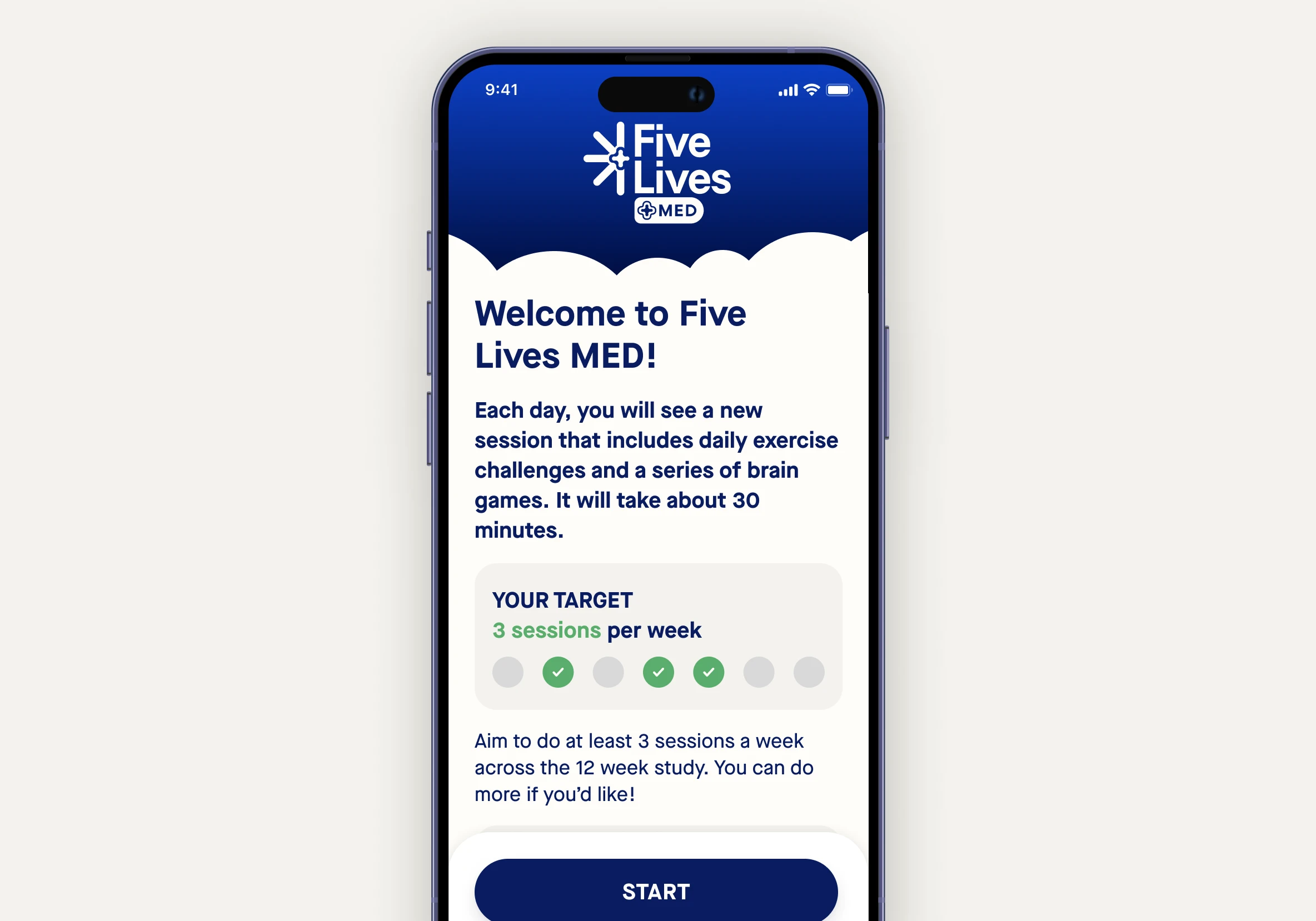
Five Lives MED is an app for mobile devices (smartphone or iPad) that combines:
Each day you will be presented with a session to follow, which will last around 30 minutes. After the daily session, you will have free access to the brain games and educational material.
We ask that you do 3 sessions per week minimum, but you can do more if you wish.
There are low risks for psychological/physical harm in this study: the Five Lives MED app is designed to be engaging and self-administered comfortably at home. For the physical exercise part of the intervention, the app provides safety instructions for each exercise: please follow these carefully. In the unlikely case of injury related to the intervention, please report the incident to your person of contact at your dedicated study site.
By participating in either group in the study, you may benefit by improving your cognitive function and physical fitness. Regardless of which group you are initially allocated to, once the study is over, you will receive free access to the Five Lives MED app for 6 months.
Your personally-identifiable information (name, address, contact details) will remain available only to the study site that contacted you. The Chief Investigator, Five Lives and our collaborative academic partner, Oxford University, will have access only to research data where any personally-identifiable data will be substituted with a study ID (such as “FLM-001”).
Only the study site will keep a secure record of which study ID corresponds to which person. This record will be kept for up to 3 years, in case you need to be contacted for whatever reason related to the study or indicate on the consent form that you give permission to be contacted for a future research study. At the end of the 3 year period, these records will be destroyed, and only the anonymised study data will remain (app usage, scores from cognitive assessments and other questionnaires, with the Study ID only).
The anonymised data from the assessments will be stored for up to 15 years on an electronic Case Report Form (eCRF) database, which is specifically designed for the secure storage of this type of data. The anonymised app data and assessment data may be used for additional analyses in future research, provided that this research has received ethical approval.
The findings from this research may be presented at scientific conferences and published in scientific articles. However, it will not be possible for you or other participants to be identified.
As sponsors of the study, Five Lives will process your data for the purpose of the research outlined above but will comply with the Data Protection Act, which requires data to be anonymised as soon as it is practical to do so.
Five Lives has procedures in place to reduce known risks of data privacy and security by adhering to ISO 27001, which ensures robust cybersecurity and data protective practices.
You have rights to access information held about you, which is in accordance with the General Data Protection Regulation (GDPR), including the right to access, correct, or request the deletion of your data. Please contact the Five Lives team (details at the end of this sheet) for more information.
Five Lives is both the sponsor of the study and main funder.
This research has received favourable ethics approval from the NHS Research Ethics Committee (insert REC committee and REC number).
If you have a concern about any aspect of this research, please contact the designated person at your study site:
If you experience technical issues with the Five Lives MED application or wish to raise any other issue directly with Five Lives, please email med.research@fivelives.health for the Research Support Team.
Thank you for reading this information and considering taking part in this research.